Pharmaceutical R&D Transformation






Client Requirement
It gives us immense pride that we have undertook a transformative project in collaboration with a prominent pharmaceutical research and development (R&D) organization. The objective was to enhance and streamline the pharmaceutical R&D process through cutting-edge technology solutions.
The client, a global pharmaceutical company with a robust R&D division, faced challenges in accelerating drug discovery, optimizing clinical trials, and managing complex data sets. The need for a comprehensive IT overhaul became evident to ensure agility, efficiency, and compliance with evolving regulatory standards.
Objective
Digitize R&D Processes
Implement digital solutions to streamline and automate various stages of drug discovery and development.
Enable Data Integration
Create a centralized platform for seamless data integration, facilitating collaboration among research teams.
Enhance Regulatory Compliance
Develop a system that ensures adherence to regulatory standards and expedites compliance processes.
Our Approach
Data Platform Deployment
- Collaborated with the client’s IT team to deploy the integrated data platform.
- Conducted thorough testing and validation to ensure data accuracy and reliability.
Workflow Automation Rollout
- Implemented the workflow automation system in a phased approach, minimizing disruptions.
- Provided training sessions for end-users to maximize adoption and utilization.
Compliance Management Integration
- Worked closely with regulatory experts to integrate and customize the compliance management system.
- Conducted regular audits to validate the system’s effectiveness in maintaining compliance.
Cloud Migration
- Executed a seamless migration of R&D applications and data to the cloud.
- Implemented security measures to safeguard sensitive research data.
Solution
SyanSoft proposed a multi-faceted approach to address the client’s challenges:
Integrated Data Platform
- Implemented a centralized data platform for seamless integration of diverse data sources.
- Utilized advanced analytics and machine learning algorithms to extract meaningful patterns and insights from the data.
Workflow Automation
- Developed a customized workflow automation system to streamline R&D processes.
- Automated data capture, analysis, and reporting, reducing manual intervention and minimizing errors.
Compliance Management System
- Integrated a robust compliance management system to ensure adherence to industry regulations.
- Regularly updated the system to align with changing regulatory requirements.
Cloud-Based Infrastructure
- Migrated key R&D applications and data to a secure and scalable cloud infrastructure.
- Improved accessibility, collaboration, and data sharing among research teams.
Results
Improved Data Insights
The integrated data platform led to improved data quality and faster access to insights, accelerating drug discovery processes.
Enhanced Workflow Efficiency
Workflow automation reduced project timelines, increased collaboration, and minimized errors, resulting in overall efficiency gains.
Stricter Regulatory Compliance
The compliance management system ensured adherence to regulatory standards, reducing the risk of non-compliance and associated penalties.
Increased Collaboration
Cloud-based infrastructure facilitated real-time collaboration among global research teams, fostering innovation and knowledge sharing.
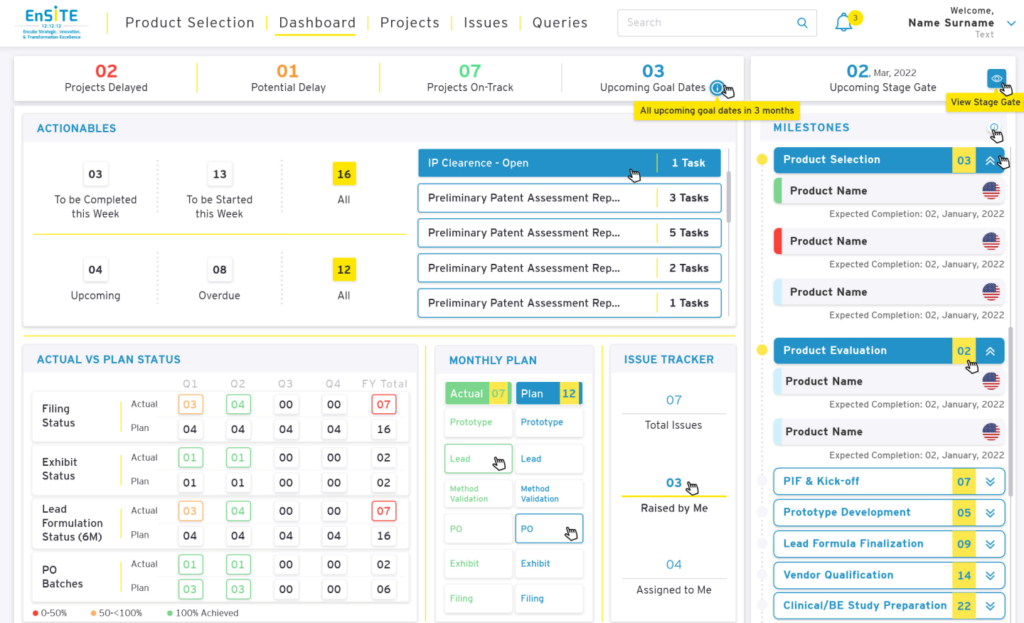

Conclusion
The collaboration between SyanSoft and the pharmaceutical R&D organization resulted in a successful transformation of their IT landscape. The integrated solutions significantly enhanced data management, workflow efficiency, and regulatory compliance, positioning the client for continued success in the dynamic pharmaceutical industry. This case study exemplifies the positive impact of leveraging cutting-edge IT solutions in the realm of pharmaceutical research and development.